Our laboratory at the Johns Hopkins University School of Medicine conducts basic and translational research aimed at better understanding the pathogenesis of multiple sclerosis (MS) and the role of the immune system in CNS health and disease. We are particularly interested in understanding the processes that drive progressive disability such as neurodegeneration and remyelination failure, with a goal of developing new therapies for MS and other autoimmune and neurodegenerative diseases.
We currently have three parallel research programs:
Metabolism as a modulator of immune, neuronal, and glial function: Previously considered part of homeostatic housekeeping, basic metabolic pathways are increasingly recognized to determine downstream function in many cell types, regulating phenotypic decisions and providing metabolites that serve as signaling molecules. We’re interested in how metabolic pathways regulate differentiation and function of peripheral immune cells, oligodendrocyte precursor cells (OPCs), microglia, and astrocytes. We hope to exploit these pathways with drugs and/or diet to prevent injury and promote repair in MS and other autoimmune conditions.
Identifying pathways by which nitric oxide (NO) and other free radicals cause neuronal and axonal damage: Although myelin is thought to be the primary target of attack in MS, neurodegeneration (injury and death of nerve cells) is nearly universal, particularly in progressive MS, and appears to be the underlying cause of permanent disability. NO and other free radicals are toxic products resulting from inflammation and chronic demyelination that contribute to neurodegeneration, but the exact mechanisms by which they cause injury are unclear. Our lab is identifying specific signaling pathways initiated by NO and other free radicals that can be targeted by drugs to produce neuroprotection. We are particularly interested in identifying druggable pathways by which NO produces mitochondrial injury.
Modulating the innate immune system in MS and other neurodegenerative diseases: Innate immune cells in the CNS – microglia and macrophages (and now astrocytes!) – appear to modulate the course of neurologic diseases, both inflammatory diseases like MS but also classic neurodegenerative diseases like Alzheimer’s and Parkinson’s. These cells take on distinct phenotypes and can be either helpful or harmful, contributing to injury or setting the stage for repair. We’re studying the mechanisms that modulate microglia and astrocyte phenotype in the brain and how to manipulate them pharmacologically.
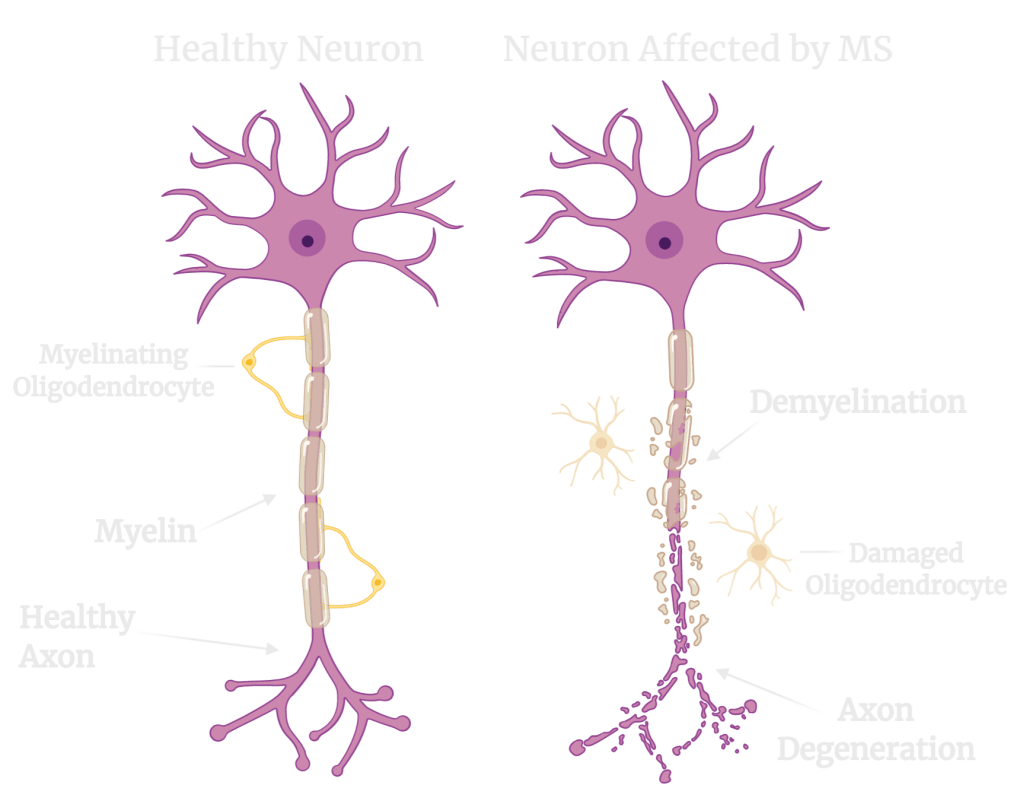
About Multiple Sclerosis

Multiple sclerosis (MS) is a chronic, inflammatory disease of the CNS that affects more than 900,000 Americans and causes physical, cognitive, and psychological disability. Although classically considered a demyelinating disease, neurodegeneration occurs nearly universally and correlates most closely with disability, likely caused by a combination of inflammatory injury and the effects of chronic demyelination. For most patients, early disease (called relapsing MS) is characterized by episodes of peripheral immune activation with invasion of the CNS causing focal inflammatory demyelination and axonal injury, while later disease (progressive MS) involves insidious progression of disability characterized by persistent innate immune activation, remyelination failure, and neurodegeneration